A representative particle is the smallest unit in which a substance naturally exists. For most elements, the representative particle is the atom. Iron, carbon, and helium are composed of iron atoms, carbon atoms, and helium atoms.
Seven elements exist in nature as diatomic molecules (H2, N2, O2, F2, Cl2, Br2, and I2), so the representative particle for these elements is a molecule. Similarly, all molecular compounds such as H2O and CO2, exist as molecules, so a molecule is also their representative particle.
For ionic compounds such as NaCl and Ca (NO3)2, the representative particle is the formula unit. A mole of any substance contains Avogadro’s number (6.02 × 1023) of representative particles.
A representative particle is the smallest unit of matter that can be broken down without changing its composition. Matter consists of three representative particles: atoms, molecules, and formula units.

Atoms and Elements
Atoms are the smallest units of matter, and different types of atoms make up different elements. They can exist by themselves or be bound together in molecules. The subatomic particles that makeup atoms are protons, neutrons, and electrons. Elements have unique properties and atomic structures.
Molecules
A molecule is a group of two or more atoms that form the smallest identifiable unit into which a pure substance can be divided while retaining the composition and chemical properties of that substance.
A molecule is two or more atoms joined by chemical bonds that form the smallest unit of a substance that retains the composition and properties of that substance. Molecules form the basis of chemistry. Molecules are labeled with an element symbol and an index with the number of atoms.
A molecule is a representative particle of molecular compounds. It is also a representative particle of diatomic elements.
Formula unit
A formula unit in chemistry is the empirical formula of any ionic or covalent network solid compound used as an independent entity for stoichiometric calculations. It is the lowest integer ratio of ions represented in an ionic compound. Examples include ionic NaCl and K2O and covalent networks such as SiO2 and C (such as diamond or graphite).
Ionic compounds do not exist as single molecules; thus, the formula unit indicates the lowest reduced ion ratio in the compound.
A chemical formula shows the types and numbers of atoms in the smallest representative unit of a substance.
Diatomic elements
Diatomic molecules are molecules composed of only two atoms, the same or different chemical elements. If a diatomic molecule consists of two atoms of the same element, such as hydrogen (H2) or oxygen (O2), then it is said to be homonuclear.
Otherwise, the molecule is said to be heteronuclear if a diatomic molecule consists of two different atoms, such as carbon monoxide (CO) or nitric oxide (NO). Bonding in a homonuclear diatomic molecule is nonpolar.
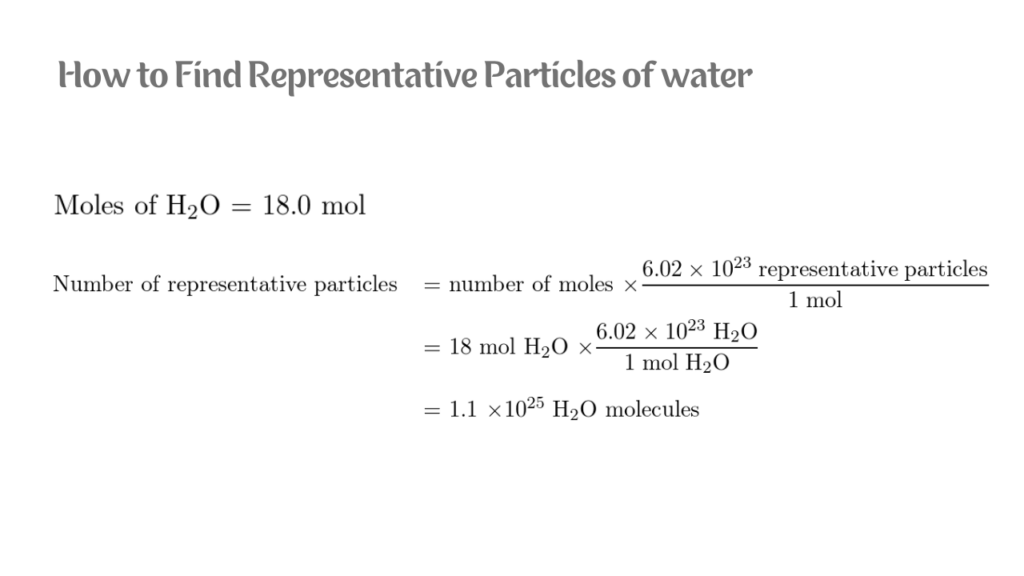
How to Find Representative Particles?
The standard unit used to represent the amount of a substance is the mole, where 1 mole contains 6.02 x 1023 particles. This quantity is referred to as Avogadro’s number.
Measure the mass of the substance in grams. Suppose that you wanted to know how many hydrogen atoms were in a mole of water molecules.
First, you would need to know the chemical formula for water, which is H2O. There are two atoms of hydrogen in each molecule of water. How many atoms of hydrogen would there be in two water molecules? There would be 2×2 = 4 hydrogen atoms.
How about in a dozen? Since a dozen is 12, there would be 12×2 = 24 hydrogen atoms in a dozen water molecules. To get the answers, (4 and 24) you had to multiply the given number of molecules by two atoms of hydrogen per molecule. Similarly, to find the number of hydrogen atoms in a mole of water molecules, the problem could be solved using conversion factors.
Two water molecules contain 4 hydrogen atoms and 2 oxygen atoms. A mole of water molecules contains 2 moles of hydrogen atoms and 1 mole of oxygen atoms.
Avogadro’s number has a value of 6.02 x 1023. Continuing the example, 2 moles of water x 6.02 x 1023 particles per mole = 1.20 x 1024 particles.
FAQs
What are the 4 types of representative particles?
A representative particle is any kind of particle such as atoms, molecules, formula units, electrons, or ions. The number 6.022 136 7 1023 is called Avogadro’s number in honor of the Italian physicist and lawyer Amedeo Avogadro who, in 1811, determined the volume of one mole of a gas.
How do you name representative particles?
For a molecular compound (H2O), a representative particle is a molecule. For an ionic compound (Na2SO4), a representative particle is a formula unit. For an atomic substance (He, Fe), a representative particle is an atom.
Is an ion a representative particle?
Representative Particle: The smallest part of a substance that retains the properties of the substance. For a molecular compound (H2O), a representative particle is a molecule. For an ionic compound (Na2SO4), a representative particle is a formula unit.
What is the representative particle for NaCl?
These particles are also known as formula units. Since one mole of NaCl contains 6.022 × 1023 formula units, therefore, the representative particles in NaCl are 6.022 × 1023 NaCl .
What are the 7 particles?
All the subatomic particles, including composite particles and fundamental particles include the following: Protons, neutrons, and electrons. six antiquarks of the same name. six leptons – electrons, electron neutrino, muon, muon neutrino, tau, and tau neutrino.
What are the 17 types of particles?
The Standard Model currently accounts for 6 quarks (up, down, strange, charm, bottom, top), 6 antiquarks, 6 leptons (electron, muon and tau, and their respective neutrinos), 6 antileptons, 13 gauge bosons (8 gluons, photon, W+, W-, Z and graviton) and 1 Higgs boson. That adds up to 38 distinct elementary particles.”